Natural Amorphous Graphite

Natural Amorphous Graphite
The term “amorphous graphite” is a contradiction in terms All graphite is crystalline by definition, therefore it is impossible for graphite to be amorphous. However, the term was applied due the anhedral (no visible crystallinity) morphology of this form of graphite. To the untrained eye a piece of amorphous graphite simply looks amorphous”, like a lump of anthracite coal. However, it is much denser than anthracite, 2.2g/cc vs. 1.7g/cc, and is soft and lubricious. A much better descriptive term for this substance would be “microcrystalline graphite” or “cryptocrystalline graphite”. Both of these geological terms are used to describe a mineral which is composed of crystals so small that they cannot be resolved with either the naked eye or through standard optical microscopy techniques. However, the material is not glassy, there are crystals present. A good analogy to more common mineral specie would be the microcrystalline form of quartz known as chert. Amorphous graphite is to flake graphite and vein graphite as chert is to crystal quartz. The photo, below right, is a polarized light micrograph of amorphous graphite showing a mineral inclusion in the fine grain amorphous graphite background mass. (Polarized Light Image courtesy of J. Crelling, SIU)
Amorphous graphite is a seam mineral, not to be confused with a vein mineral. It is formed by the metamorphism of previously existing anthracite coal seams. Proto-coal organic carbon is deposited and converted into anthracite coal followed by low-grade metamorphism of the coal. This results in the formation of microcrystalline graphite. Regions, where this process occurs, may consist of zones where anthracite deposits occur adjacent to graphite deposits. This phenomenon is indicative of localized metamorphism or contact metamorphism.
Contact metamorphism is metamorphism that is the result of localized contact between the agent of metamorphism (magma intrusion, local tectonic stress, etc.) and the body being metamorphosed A good example of this type of graphite formation is the amorphous graphite deposits of Sonora, Mexico. In these deposits the graphite affecting agent has been identified as swarms of dikes that intruded the area.Dikes are intrusions of magma that are tabular in shape, and cut across the structure of the country rocks. The illustration is a schematic of such a contact between a coal seam and its intruded dike. Although the coal bed illustrated is shown “parallel’ to the local bedding, most if not all anthracite seams occur in folded strata, which are indicative of the semi-metamorphic nature of this variety of coal.
Note that the formation of amorphous graphite is not limited to the contact metamorphism agent only. Regional metamorphism may occur as when a large intrusion such a batholith or stock intrudes beneath or adjacent to structures containing the right type of precursor carbon.
Seam graphite is typically higher in ash than other forms of natural graphite. Since the proto-carbon is deposited contemporaneously with other mineral matter that flows into swamps, bogs, deltas, and other “coal producing” environments. Since the ‘system’ is more or less closed to the exchange of mass with the country rock, the mineral matter (ash) that was present in the coal remains in the graphite. This mineral matter may be either “free”, i.e., “mechanically” attached particulates such as quartz, or may consist of clay-like minerals that are intimately associated with the carbon.
Amorphous graphite is the most abundant form of natural graphite. The macroscopic morphology of this graphite variety is called “massive” which is indicative of its homogeneous non-stratified, microcrystalline structure. The long-range order common to crystalline substances is minimal in this graphite variety. Crystallinity is poor as is indicated by the fact that none of the common crystal forms (crystal faces) visible in flake and vein graphite are visible in amorphous graphite. Based on “degree of graphitization” as determined by X-ray diffraction studies, amorphous graphite typically shows “graphite content” of 20-40 percent as compared to 90+ percent for other natural graphite types.
The development of long-range crystallographic order requires that some degree of the pre-crystalline order be present in the proto-carbon and that the energy supplied to this carbon be sufficient to promote graphitization. In the case of amorphous graphite, it is apparent that only minimal pre-ordering of proto-crystallographic domains occurred.
Amorphous graphite is extracted using conventional coal-type mining techniques. Amorphous graphite is typically lower in purity than other natural graphite. This is due to the intimate contact between the graphite “micro crystals” and the mineral ash with which it is associated. This close graphite/ash association makes floatation and other density and chemical based separation techniques inefficient if not impossible. Commercial grades of amorphous graphite are available from 75-85% purity in particle sizes from 4-inch lumps to 3-micrometer powder.
Amorphous graphite tends to be much less reflective in both large a small grained sizes. Therefore, it has a darker color, bordering on black, while other natural graphite has a color closer to “silver-gray”. This makes amorphous graphite useful in coatings and other products, which require less reflectance. Also, this graphite variety is typically lower in cost than other types but is still lubricious, conductive, and chemically stable.
Amorphous graphite is used in many lubricant products especially greases, forging lubricants, etc. In applications where higher ash contents are acceptable or preferred this type of graphite is a good choice.
Certain applications such as mechanical seals require a certain degree of rubbing abrasion to provide the necessary “lapping” or cleaning of opposing rubbing surfaces. The ash content of amorphous graphite can provide the right balance of gentle abrasion required to perform this function. By selecting a properly sized amorphous graphite powder the user can control the size of the ash.
Asbury Carbons has literally hundreds of grades of amorphous graphite available. Grades are categorized based on particle size and purity and can be customized to fit any requirement. With many years of experience in the grinding and sizing of amorphous graphite, we can modestly state that we are experts at providing the right amorphous graphite for the job.
The small crystal sizes of the particles give it the name “Amorphous Graphite”. Amorphous Graphite has a significantly lower pricing than Flake graphite and is used in fields such as pencils, brake pads, rubber additives, etc.
The RAHA Gilsonite Co. with many years experience in selecting, processing and distributing Amorphous Graphite to the Foundry, Refractory, and Chemical and industries. Amorphous is the least “graphitic” of the natural graphites. The term “amorphous” is a misnomer since this material is in fact truly crystalline.
Amorphous Graphites are typically lower in purity than flake graphites and their method of extraction is like that of coal and commercial grades centre around 80 to 85% Carbon.
Amorphous Graphite tends to be much less reflective in both coarse and fine-grained sizes and has a darker colour, bordering on black whilst higher purity flake has a colour closer to silver grey.
It has a typically lower value than flake but still has valuable lubrication properties, is conductive and is a very stable product used in coatings, refractory blends, and chemical processes.
Application of Amorphous Graphite
Amorphous graphite is the least graphitic of the natural graphites. However, the term “amorphous” is a misnomer since the material is still crystalline. Amorphous graphite is found as minute particles in beds of mesomorphic rocks such as coal, slate or shale deposits. The graphite content ranges from 25% to 85% dependent on the geological conditions.
Amorphous graphite is extracted using conventional mining techniques.
Due to its high-temperature stability and chemical inertness graphite is a good candidate for a refractory material. It is used in the production of refractory bricks and in the production of “Mag-carbon” refractory bricks (Mg-C.) Graphite is also used to manufacture crucibles, ladles, and moulds for containing molten metals. Additionally, graphite is one of the most common materials used in the production of functional refractories for the continuous casting of steel. In this application, graphite flake is mixed with alumina and zirconia and then isostatically pressed to form components such as stopper rods, subentry nozzles and ladle shrouds used in both regulating flows of molten steel and protecting against oxidation. This type of material may also be used as shielding for pyrometers.
In the production of iron, graphite blocks are used to form part of the lining of the blast furnace. Its structural strength at temperature, thermal shock resistance, high thermal conductivity, low thermal expansion and good chemical resistance are of paramount importance in this application.
The electrodes used in many electrical metallurgical furnaces are manufactured from graphite such as the electric arc furnaces used for processing steel.
Chemical Industry
There are many high temperature uses for graphite in the chemical industry such as in the production of phosphorus and calcium carbide in arc furnaces. Graphite is used as anodes in some aqueous electrolytic processes such as in the production of halogens (chlorine and fluorine.)
Nuclear Industry
High purity electrographite is used in large amounts for the production of moderator rods and reflector components in nuclear reactors. Their suitability arises from their low absorption of neutrons, high thermal conductivity and their high strength at temperature.
Electrical Applications
The main application for graphite as an electrical material is in the manufacture of carbon brushes in electric motors. In this application, the performance and lifetime of the component is very dependent on grade and structure.
Mechanical Applications
Graphite is used widely as an engineering material over a variety of applications. Applications include piston rings, thrust bearings, journal bearings, and vanes. Carbon based seals are used in the shafts and fuel pumps of many aircraft jet engines.
Other Application
Amorphous graphite has applications in:
- Lubricant additives
- Friction Materials
- Refractories
- Paint production
- Rubber & Polymer Composites
- Metallurgy
- Metal Covers
- Thread Compounds
- Drilling Mud Additives
- Pencil production
Mesh Size of Amorphous Graphite
Amorphous Graphite has numerous applications due its fundamental softness, including in lubricant formulations and as an additive to structural materials and metallic alloys. We provide natural amorphous graphite as a powder at –100 mesh, –200 mesh, and –325 mesh, as a grain with size ranges of 0-3mm, 0-6mm, 0-15mm, 1-5mm, 2-5mm, 1-6mm, and as briquettes with specifications of 45x38x25mm, 45x45x20mm, 30x25x20mm, 25x20x16mm, all available in the following purities: 70%, 75%, 78%, 80%, 83%, 85%, 88%, and 90%.
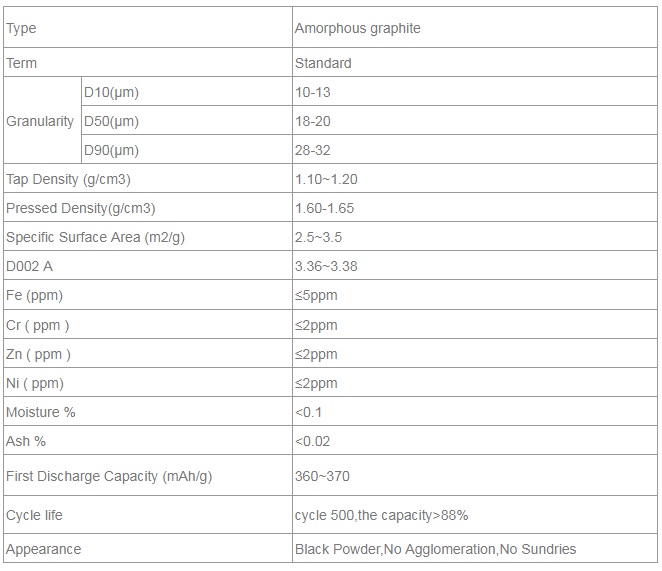
Standard of amorphous graphite
Natural Amorphous Graphite is generally immediately available in most volumes. We produce to many standard grades when applicable, including Mil Spec (military grade); ACS, Reagent and Technical Grade; Food, Agricultural and Pharmaceutical Grade; Optical Grade, USP and EP/BP (European Pharmacopoeia/British Pharmacopoeia) and follows applicable ASTM testing standards. Typical and custom packaging is available. Additional technical, research and safety (MSDS) information is available as is a Reference Calculator for converting relevant units of measurement.
Packing of Amorphous Graphite
lump form like rock packed in the 500~1000kg jumbo bag
200 mesh packed in the 500~1000kg jumbo bag
300 mesh packed in the 500~1000kg jumbo bag
30-40 mesh packed in the 500~1000kg jumbo bag
100 mesh packed in the 500~1000kg jumbo bag
300 mesh packed in the 25kg pp bag
200 mesh packed the 25kg multi-paper bag
200 mesh packed 50lbs multi-paper bag
30-40 mesh packed pp bag on the pallet
Bulk on vessel
Specification of Amorphous Graphite