Gilsonite Asphalt
Scientists improve method to pull greenhouse gas from natural gas streams
A Rice University laboratory has improved its method to turn plain asphalt into a porous material that can capture greenhouse gases from natural gas.
In research detailed this month in Advanced Energy Materials, Rice researchers showed that a new form of the material can sequester 154 percent of its weight in carbon dioxide at high pressures that are common at gas wellheads.
Raw natural gas typically contains between 2 and 10 percent carbon dioxide and other impurities, which must be removed before the gas can be sold. The cleanup process is complicated and expensive and most often involves flowing the gas through fluids called amines that can soak up and remove about 15 percent of their own weight in carbon dioxide. The amine process also requires a great deal of energy to recycle the fluids for further use.
“It’s a big energy sink,” said Rice chemist James Tour, whose lab developed a technique last year to turn asphalt into a tough, sponge-like substance that could be used in place of amines to remove carbon dioxide from natural gas as it was pumped from ocean wellheads.
Initial field tests in 2015 found that pressure at the wellhead made it possible for that asphalt material to adsorb, or soak up, 114 percent of its weight in carbon at ambient temperatures.
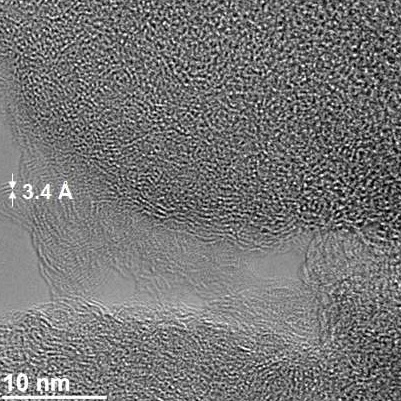
A transmission electron microscope image of Gilsonite-derived asphalt shows a micropore in the structure. The pores sequester carbon dioxide under common pressures at natural gas wellheads.
Tour said the new, improved asphalt sorbent is made in two steps from a less expensive form of asphalt, which makes it more practical for industry.
“This shows we can take the least expensive form of asphalt and make it into this very high surface area material to capture carbon dioxide,” Tour said. “Before, we could only use a very expensive form of asphalt that was not readily available.”
The lab heated a common type asphalt known as Gilsonite asphalt at ambient pressure to eliminate unneeded organic molecules, and then heated it again in the presence of potassium hydroxide for about 20 minutes to synthesize oxygen-enhanced porous carbon with a surface area of 4,200 square meters per gram, much higher than that of the previous material.

A scanning electron microscope image shows micropores in carbon capture material derived from common asphalt. The material created at Rice University sequesters 154 percent of its weight in carbon dioxide at 54 bar pressure, a common pressure at wellheads.
The Rice lab’s initial asphalt-based porous carbon collected carbon dioxide from gas streams under pressure at the wellhead and released it when the pressure was released. The carbon dioxide could then be repurposed or pumped back underground while the porous carbon could be reused immediately.
In the latest tests with its new material, Tours group showed its new sorbent could remove carbon dioxide at 54 bar pressure. One bar is roughly equal to atmospheric pressure at sea level, and the 54 bar measure in the latest experiments is characteristic of the pressure levels typically found at natural gas wellheads, Tour said.
Advances gilsonite asphalt-based filter to sequester greenhouse gas at the wellhead
A dab of water aids carbon capture
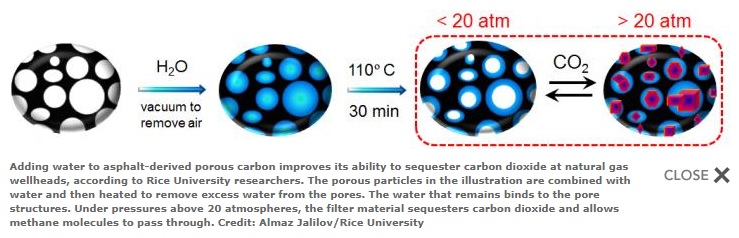
This illustration shows how porous particles in Gilsonite asphalt-derived porous carbon are combined with water and then heated to remove excess water from the pores; the water that remains binds to the pore structures. Under pressures above 20 atmospheres, the filter material sequesters carbon dioxide but allows methane molecules to pass through.
Scientists at Rice University have found a way to make their asphalt-based sorbents better at capturing carbon dioxide from gas wells: just add water.
The Rice lab of chemist James Tour discovered that treating grains of inexpensive Gilsonite asphalt with water allows the material to adsorb more than two times its weight of the greenhouse gas. The treated asphalt also selects carbon dioxide over valuable methane at a ratio of more than 200-to-1.
The material performs well at ambient temperatures and under the pressures typically found at wellheads. When the pressure abates, the material releases the carbon dioxide, which can then be stored, sold for other industrial uses or pumped back downhole.
Adding water to asphalt-derived porous carbon improves its ability to sequester carbon dioxide at natural gas wellheads, according to Rice University researchers. The porous particles in the illustration are combined with water and then heated to remove excess water from the pores. The water that remains binds to the pore structures. Under pressures above 20 atmospheres, the filter material sequesters carbon dioxide and allows methane molecules to pass through.
Natural Gas at the wellhead typically contains between 3% and 7% carbon dioxide, but at some locations, it may contain up to 70%. Oil and gas producers traditionally use one of two strategies to sequester this carbon dioxide: physically, through the use of membranes or solid sorbents like zeolites or porous carbons; or chemically, through filtering with the liquid amine, a derivative of ammonia.
But both these methods have drawbacks. Physical filters have a hard time differentiating between carbon dioxide and methane molecules, which are nearly identical in size (3.3 angstroms vs 3.8 angstroms) and polarizability (important to bonding characteristics). Chemical approaches have better selectivity but are more expensive and corrosive, and they require a large input of energy and large equipment. Despite their high selectivity, amines capture only 13% by weight of carbon dioxide and need superheated steam to release it, while the Rice team’s system can capture more than 200% by weight.
The new Rice material features the selectivity of amines, but with a much higher uptake of carbon dioxide and no thermal requirements, Tour said. Coating the pore surfaces with water adds weak chemical absorption and high selectivity while retaining the material’s strong physical adsorption.
“This is known as a pressure-swing adsorption system, which is easy to implement due to its small size, and there’s no need for heating since it works with the inherent pressure in the gas well,” Tour said.
Water in Gilsonite forms a hydrate within the pore microstructures that greatly increases the binding selectivity of carbon dioxide over methane, according to the researchers. While the grains’ micropores, at 23 angstroms, are far larger than the target molecules, adding water tightens the pores and decreases the pore volume through which the molecules must travel. The prepared Gilsonite Asphalt has a surface area of 4200m2 per gram, so adding water still leaves plenty of room to capture carbon dioxide, Tour said.
Over multiple testing cycles at various pressures and temperatures between freezing and 50°C, degradation of the material was reportedly negligible. The researchers found that about 1% weight of the water content was lost during cycling but predicted that the water content of natural gas itself would likely replace that.

