Gilsonite Properties

Gilsonite Properties
Gilsonite greatly resembles asphalt. of which it is considered a species by many authors. Asphalt readily becomes softened by the warmth of the hand or the rays of the sun. However, Gilsonite is not affected by heat below the boiling point of water. All gilsonite does not have the same melt point, varying widely from about 230° F to 500° F, depending upon the particular vein from which the sample has been taken. Some of the larger veins contain more than one type of gilsonite. giving evidence of the vicissitudes that have affected the material during vein filling. polymerization and weathering. When fused, Gilsonite melts like tar and seals out the air that would support combustion.
Gilsonite having a melting point of approximately from 150C up to 220. (Ball and Ring method) and ash of solved and, therefore, is poorly suited as a saturating material for brake linings for instance, where a considerable proportion of the colloidal material is left on the surface when impregnation is attempted.
Natural asphalt or mineral bitumen is a sedimentary rock formed in wide, low-lying equatorial swamps crossed by large rivers and covered by forests of primitive animals. Here, the remains of animals saved from biodegradation and oxidation by mud and water. Gilsonite is usually black in color but sometimes it occurs as a brownish-black color. There are four broad ranks or types of Gilsonite depending upon its age. Commencing with the youngest and lowest carbon content.
Gilsonite is not an asphalt if the term “asphalt” be restricted to its usual and primary meaning. gilsonite belongs to the family of substances known as asphaltites. which includes Grahamite, glance pitch, etc. Asphaltites are quite as different from asphalt as asphalt is from petroleum, or as petroleum is from natural gas. While gas, petroleum, asphalts, and asphaltites belong to the hydrocarbon series. they each constitute a distinct. though the overlapping sequence of state in which the composite. molecular arrangement. and other properties seem to vary chiefly with the degree of polymerization or fractionation. and the molecular history of the member of the series being considered.
Gilsonite is completely dissolved in the ordinary solvents such as gasoline or benzol unless, for instance, it is refluxed with these solvents at a high temperature. This is probably due to the fact that Gilsonite is highly colloidal and therefore, tends to resist complete smooth solution.
Gilsonite being a natural material varies in composition depending upon its origin, history of formation and the geographical region and the presence of other minerals such as sulphur, phosphorus, and others. So little wonder gilsonite does not have a specific composition. In general, it is Hydrocarbon and some of the carbon will be in the form of volatiles which evaporates readily as in Gilsonite. There is also inert material either mineral or within the carbon which forms ash. Complex chemical compounds are also formed during natural bitumen formation and some of these may have toxic properties. Gilsonite technology is therefore quite complex and important in its utilization.
Regards toxicity and environmental pollution, present day technology are well developed in reducing impacts to within acceptable levels.
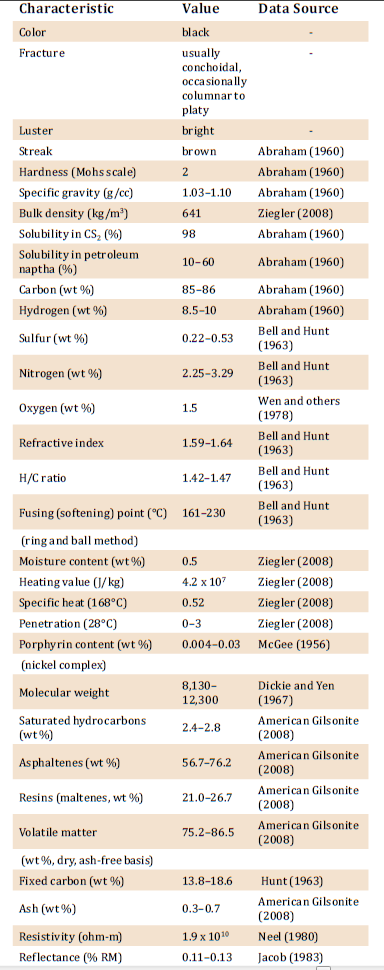
Physical and Chemical Characterization of Gilsonite
- Black color
- Shiny
- Similar in appearance to the mineral obsidian
- Highly brittle and easily crushed into dark brown powder
- Melting temperature between 160 and 220 C
- High-content of Asphaltene
- High solubility in organic solvents
- High purity and compatibility
- High- content of nitrogen
The low specific gravity of Gilsonite powder helps improve its ability to control lost circulation. However, this feature can also cause the additive to separate to the top of thin slurries and slurries containing dispersants. Adding 2% or more bentonite to the slurry will help prevent separation.
Gilsonite Additive Can Provide the Following Benefits:
When perforated, it is shatter-resistant.
It does not significantly affect the setting time of cement.
Gilsonite additive can provide higher strength than heavier additives with high water requirements.
Chemical Properties of Gilsonite
Gilsonite is a complex system of different constituents, made of hydrocarbons and heteroatoms. After fractionation of the Gilsonite by specific solvents, four main chemical families are obtained: saturates, aromatics, resins, and asphaltenes. The association of asphaltene sheets (highly polycondensed pseudo monomers of a carbon black-bone, chemical functions, and heteroatoms surrounded by aliphatic chains) leads to the formation of microstructures or “micelles”. They can also form aggregates.
Gilsonite is included in a class of solid bitumens known as asphaltites. Gilsonite deposits are located in eastern Utah in the United States. They are different from other asphaltites because of their:
- High asphaltene content
- High solubility in organic solvents
- High purity and consistent properties
- High molecular weight
- High nitrogen content
Gilsonite is available in different grades categorized by softening point. The softening point is used as an approximate guide to melt viscosity and behavior in solution.

